UMONIUM38® PROBES wipes - achieve HLD in 5 seconds using this wipe
UMONIUM38® PROBES wipes - achieve HLD in 5 seconds using this wipe
The Advanced, Rapid High-Level Disinfectant Designed Specifically for Your Valuable Ultrasound Equipment.
Inadequate disinfection of ultrasound probes serves as a potential vector for Healthcare-Associated Infections (HAIs), risking patient morbidity, extended hospital stays, and increased costs. For semi-critical probes—devices that contact mucous membranes or non-intact skin, such as transvaginal, transrectal, and Trans-Oesophageal Echocardiography (TOE) probes—High-Level Disinfection (HLD) is non-negotiable to prevent pathogen transmission.
UMONIUM38® PROBES wipes offers a patented high-level disinfectant solution formulated specifically for ultrasound transducers, achieving the critical balance between powerful antimicrobial efficacy, patient safety, and material protection
Technical Efficacy and Workflow Integration
UMONIUM38® PROBES wipes redefine HLD standards by combining rapid action with broad-spectrum kill power, ensuring compliance and maximising workflow efficiency in busy clinical environments like Radiology Departments and Emergency Departments.
Key Technical Advantages:
| Feature | Technical Detail | Supporting Data |
| 5-second High level disinfection using this wipe |
Achieves full high-level disinfection on endocavity probes after 5 seconds of wiping, offering more time between patients compared to traditional methods. Shorter wiping contact time (5 seconds) improves workflow efficiency. |
Shorter wiping contact time (5 seconds) improves workflow efficiency. |
| Superior Antimicrobial Efficacy |
Provides broad-spectrum efficacy against bacteria, fungi, and viruses. Ensures elimination of resistant pathogens, including MRSA, HPV, and Mycobacterium tuberculosis. |
Achieves a >5-log reduction in microbial load. Effective against non-enveloped viruses (HPV, norovirus). |
| Residue-Free Formulation |
Leaves no residue on treated surfaces, eliminating the need for extensive rinsing (Step 5 of the protocol). This feature saves time and reduces the risk of recontamination or potential interference with ultrasound image quality. |
NO rinsing required, except with direct contact with mucous membranes. Simple protocol improves workflow efficiency |
| Material Compatibility & Neutral pH |
Specifically formulated with a neutral pH of 7 to be compatible with the sensitive materials used in ultrasound transducers. Protects equipment integrity, extending the lifespan of expensive medical devices. |
Does not harm equipment, maintaining optimal imaging performance. |
| Safety and User Comfort |
Free from toxic fumes. Requires minimal respiratory irritation potential and no specialised ventilation systems. Suitable for implementation in existing reprocessing areas. |
Meets requirements for implementation without costly ventilation upgrades. |
Unparalleled Safety Validation
UMONIUM38® PROBES wipes is validated for use in the most sensitive clinical environments, supporting applications across urological, cardiological, transoesophageal, ophthalmological, and ENT examinations.
Validated for Sensitive Environments:
- IVF and Gynaecological Procedures: The product is validated as safe for use even in the IVF environment and perfectly suitable for gynaecological examinations.
- Mouse Embryo Assay (MEA) Test: The MEA test demonstrated the total innocuity of UMONIUM38® PROBES wipes environments for embryonic cells, widely used to detect toxicity that may contact gametes or embryos.
- Human Sperm Survival Assay (HSSA) Test: The HSSA test showed a complete absence of effect on the motility of sperm when exposed to the disinfected environment.
Regulatory Compliance:
UMONIUM38® PROBES wipes meets or exceeds stringent international standards for high-level disinfection.
- The product is compliant with MDD 93/42/CEE relating to medical devices and is in the process of transitioning to MDR 2017/42/CEE
- Efficacy is validated according to extensive EN standards, including EN 16615, EN 13727, EN 14476 (virucidal activity), and is active against Clostridioides difficile according to EN 17846:2023.
- Quality and traceability are guaranteed by ISO 9001:2015 and EN ISO 13485:2016.
Technical Composition Overview
The patented formulation is based on a quaternary ammonium compound combined with synergistic ingredients, providing superior disinfection performance. The active ingredients work by disrupting the cell membranes of microorganisms, leading to cell death.
| Efficacy Area | Key Organisms Targeted | Log Reduction Achieved |
| Bactericidal Activity | MRSA, Mycobacterium tuberculosis (TB), Gram-positive/negative bacteria. | Achieves >5-log reduction. |
| Virucidal Activity | Non-enveloped viruses (HPV, Norovirus) and enveloped viruses (HIV, HBV, HCV, influenza). | Provides >4-log reduction in viral infectivity. |
| Fungicidal Activity | Candida species (including C. auris), Aspergillus and other moulds. | Achieves >4-log reduction. |
| Sporicidal Activity | Clostridioides difficile, Bacillus subtilis, Aspergillus brasiliensis. | Meets EN 17846:2023 requirements. |
Maximise Longevity and Value
UMONIUM38® PROBES wipes provides operational cost efficiency through its remarkable stability and shelf life:
- Long Shelf Life: Remains effective for 3 years after opening when properly stored and handled, offering significant cost efficiency compared to single-use alternatives.
- Dirty Condition Efficacy: Tests performed showed that the product retains all its efficacy even in the presence of dirt.
- Competitive Value: When evaluating the total cost of ownership—including contact time, staff safety, and equipment longevity—UMONIUM38® PROBES wipes offers a highly competitive value compared to traditional options like glutaraldehyde.
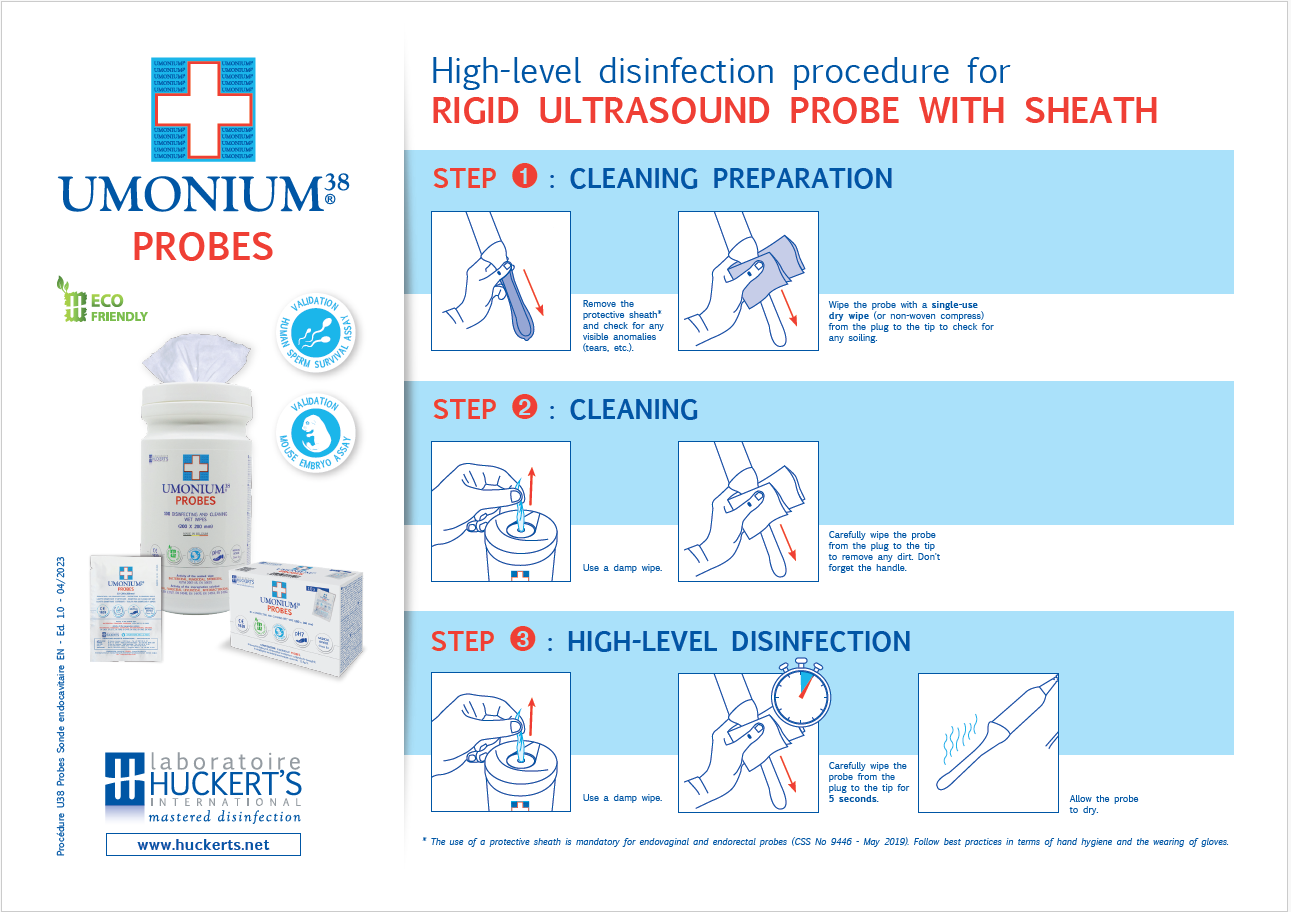
Disinfection Instructions (Achieving HLD on Endocavity Probes):
- Pre-cleaning: Remove visible soil or gel using a soft, lint-free cloth.
- Cleaning: Wipe the probe (plug to tip, including handle/cable) with a UMONIUM38® wipe to remove dirt.
- High Level Disinfection: Take a new UMONIUM38® PROBES wipe and carefully wipe the probe from the plug to the tip.
- Contact Time: Wipe ofr 5 seconds and let dry to ensure complete High-Level-Disinfection.
- Post-disinfection: No rinsing is required.
- Documentation: Record the process according to your facility's protocols.
Share